Abstract
Clonal hematopoiesis (CH) increases the risk of hematological malignancies and other adverse outcomes. The most common driver mutations occur in DNMT3A and TET2. However, it is still unclear how mutant DNMT3A and TET2 perturb human stem/progenitor cell homeostasis allowing mutant clones to expand. Studies of human CH have been limited by availability of samples from subjects without co-existing malignancy and high-precision methods for comparing mutant and wild-type (WT) cells in the same individual.
To address this gap, we screened 195 bone marrow (BM) samples collected from subjects undergoing hip replacement surgery for CH by targeted DNA sequencing. As DNMT3A and TET2 regulate DNA methylation and gene expression, we employed multiomic single-cell analysis with TARGET-seq to combine single-cell genotyping with transcriptome sequencing on FACS index sorted cells. We developed an improved method, TARGET-seq+, which provides greater sensitivity transcript detection, resulting in a median of 4500 genes/cell with high-sensitivity genotyping (95% of cells genotyped; 7-11% allele dropout rate).
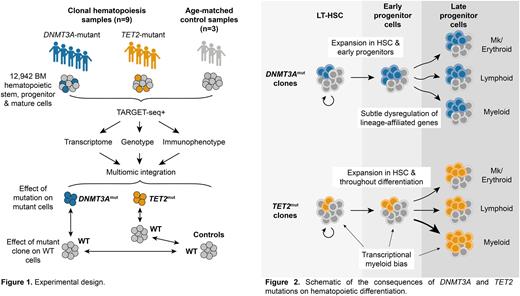
We performed TARGET-seq+ on 12,942 BM stem, progenitor and mature cells from 9 CH samples with predicted loss-of-function mutations (4 single mutation cases & 5 multiple mutation cases) and 3 normal controls (Fig. 1). Of these, 87% of cells passed quality controls and were successfully genotyped.
Integrated transcriptomic and genotyping analysis showed DNMT3Amut and TET2mut cells occupy a common differentiation trajectory with WT cells. Within DNMT3A-mutant samples, the mutant clone constituted 5-70% of transcriptomic and immunophenotypic long-term hematopoietic stem cells (LT-HSC). There was a further 1.5 to 1.7-fold expansion of DNMT3Amut cells in early erythro-megakaryocytic multipotent progenitors and lympho-myeloid multipotent progenitors (LMPP) compared to LT-HSC. Across most samples, clone size was maintained throughout differentiation with no evidence of differentiation delay or lineage bias. Differential expression analysis revealed subtle cell type-specific differences between WT and mutant cells, with upregulation of megakaryocytic lineage markers in mutant LMPP and downregulation of erythroid lineage markers in mutant erythroid progenitors.
TET2mut clones were also identified in LT-HSCs at a clone size of 1-21% but expanded further in multiple downstream progenitors. Maximal expansion (2-12 fold) was seen in the myeloid lineage, but clones also expanded in the erythroid, megakaryocytic and lymphoid lineages (mean clone size 2 to 5-fold larger than in LT-HSC). Comparison of mutant and WT granulocyte-monocyte progenitors (GMP) identified upregulation of myeloid lineage-affiliated transcription factors, (e.g. CEBPD and IRF8) in TET2mut cells. Increased expression of myeloid gene signatures was also seen within TET2mut HSC and LMPP, suggesting myeloid lineage bias arises early in differentiation. Concordantly, TET2mut HSC were shifted towards a more differentiated state in pseudotime and downregulated LT-HSC signatures.
In one sample with biallelic TET2 mutations, the TET2 single and double mutant clones were each detected in only 1 out of 109 (0.9%) CD90+CD49f+ LT-HSCs. However, the double mutant clone expanded dramatically in the myeloid lineage, contributing to >50% of cells within GMP, dendritic cell progenitor and neutrophil/monocyte precursor compartments, consistent with frequent detection of biallelic TET2 mutations in myeloid leukemias.
Comparison of gene expression between differentiation stage-matched WT cells in CH samples and in age-matched normal controls allowed us to identify the impact of CH clones on WT cells, co-existing within the same BM micro-environment. WT HSCs in DNMT3A and TET2 mutant samples showed enhanced expression of inflammatory, quiescence and chemokine gene signatures compared to WT HSCs in non-CH samples.
In summary, we present the first detailed analysis of the impact of DNMT3A and TET2 mutations on hematopoiesis in humans with unperturbed CH. The selective advantage of DNMT3A-mutant clones occurs primarily at the level of HSC and early progenitors, whereas TET2-mutant clones expand not only in HSC but also further throughout differentiation (Fig. 2). There is a non-cell autonomous impact on WT HSCs in CH. Our work provides a foundation for understanding the mechanistic basis of pre-leukemic clonal expansion.
Disclosures
Vyas:Celgene: Honoraria, Research Funding; Abbvie: Honoraria; Astellas: Honoraria; Daiichi Sankyo: Honoraria; JAZZ: Honoraria; Pfizer: Honoraria; Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal